Page 203 - Instant notes
P. 203
Rate laws in action 189
where k −1 is the desorption rate constant. At equilibrium the rates of adsorption and
desorption must equal, so:
k 1 p A(1−θ)=k −1θ
and
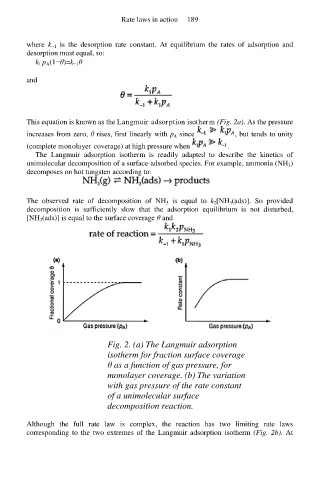
This equation is known as the Langmuir adsorption isotherm (Fig. 2a). As the pressure
increases from zero, θ rises, first linearly with p A since , but tends to unity
(complete monolayer coverage) at high pressure when .
The Langmuir adsorption isotherm is readily adapted to describe the kinetics of
unimolecular decomposition of a surface-adsorbed species. For example, ammonia (NH 3)
decomposes on hot tungsten according to:
The observed rate of decomposition of NH 3 is equal to k 2[NH 3(ads)]. So provided
decomposition is sufficiently slow that the adsorption equilibrium is not disturbed,
[NH 3(ads)] is equal to the surface coverage θ and
Fig. 2. (a) The Langmuir adsorption
isotherm for fraction surface coverage
θ as a function of gas pressure, for
monolayer coverage. (b) The variation
with gas pressure of the rate constant
of a unimolecular surface
decomposition reaction.
Although the full rate law is complex, the reaction has two limiting rate laws
corresponding to the two extremes of the Langmuir adsorption isotherm (Fig. 2b). At