Page 28 - Instant notes
P. 28
Physical Chemistry 14
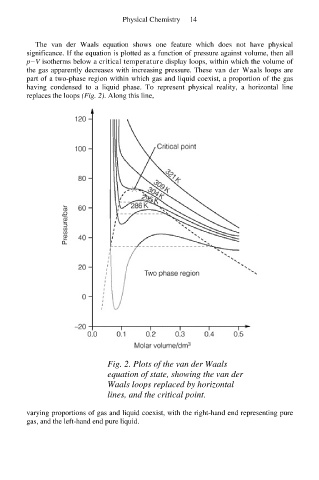
The van der Waals equation shows one feature which does not have physical
significance. If the equation is plotted as a function of pressure against volume, then all
p−V isotherms below a critical temperature display loops, within which the volume of
the gas apparently decreases with increasing pressure. These van der Waals loops are
part of a two-phase region within which gas and liquid coexist, a proportion of the gas
having condensed to a liquid phase. To represent physical reality, a horizontal line
replaces the loops (Fig. 2). Along this line,
Fig. 2. Plots of the van der Waals
equation of state, showing the van der
Waals loops replaced by horizontal
lines, and the critical point.
varying proportions of gas and liquid coexist, with the right-hand end representing pure
gas, and the left-hand end pure liquid.