Page 24 - Instant notes
P. 24
Physical Chemistry 10
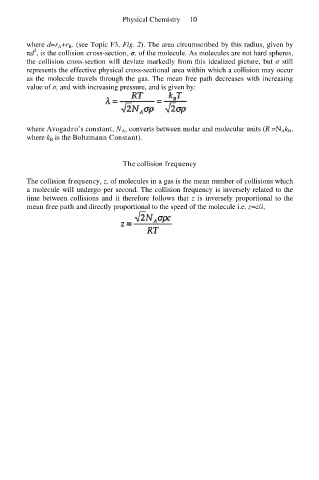
where d=r A+r B. (see Topic F3, Fig. 2). The area circumscribed by this radius, given by
2
πd , is the collision cross-section, σ, of the molecule. As molecules are not hard spheres,
the collision cross-section will deviate markedly from this idealized picture, but σ still
represents the effective physical cross-sectional area within which a collision may occur
as the molecule travels through the gas. The mean free path decreases with increasing
value of σ, and with increasing pressure, and is given by:
where Avogadro’s constant, N A, converts between molar and molecular units (R =N Ak B,
where k B is the Boltzmann Constant).
The collision frequency
The collision frequency, z, of molecules in a gas is the mean number of collisions which
a molecule will undergo per second. The collision frequency is inversely related to the
time between collisions and it therefore follows that z is inversely proportional to the
mean free path and directly proportional to the speed of the molecule i.e. z=c/λ,