Page 22 - Instant notes
P. 22
Physical Chemistry 8
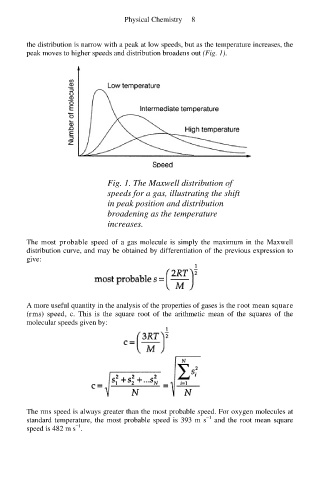
the distribution is narrow with a peak at low speeds, but as the temperature increases, the
peak moves to higher speeds and distribution broadens out (Fig. 1).
Fig. 1. The Maxwell distribution of
speeds for a gas, illustrating the shift
in peak position and distribution
broadening as the temperature
increases.
The most probable speed of a gas molecule is simply the maximum in the Maxwell
distribution curve, and may be obtained by differentiation of the previous expression to
give:
A more useful quantity in the analysis of the properties of gases is the root mean square
(rms) speed, c. This is the square root of the arithmetic mean of the squares of the
molecular speeds given by:
The rms speed is always greater than the most probable speed. For oxygen molecules at
−1
standard temperature, the most probable speed is 393 m s and the root mean square
−1
speed is 482 m s .