Page 21 - Instant notes
P. 21
Molecular behavior in perfect gases 7
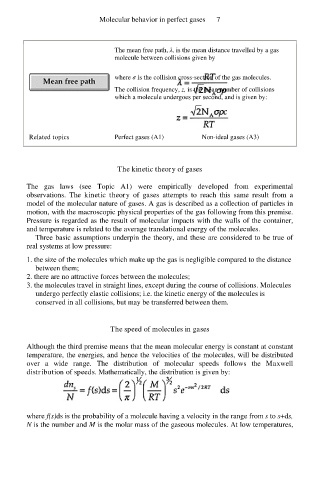
The mean free path, λ, is the mean distance travelled by a gas
molecule between collisions given by
where σ is the collision cross-section of the gas molecules.
The collision frequency, z, is the mean number of collisions
which a molecule undergoes per second, and is given by:
Related topics Perfect gases (A1) Non-ideal gases (A3)
The kinetic theory of gases
The gas laws (see Topic A1) were empirically developed from experimental
observations. The kinetic theory of gases attempts to reach this same result from a
model of the molecular nature of gases. A gas is described as a collection of particles in
motion, with the macroscopic physical properties of the gas following from this premise.
Pressure is regarded as the result of molecular impacts with the walls of the container,
and temperature is related to the average translational energy of the molecules.
Three basic assumptions underpin the theory, and these are considered to be true of
real systems at low pressure:
1. the size of the molecules which make up the gas is negligible compared to the distance
between them;
2. there are no attractive forces between the molecules;
3. the molecules travel in straight lines, except during the course of collisions. Molecules
undergo perfectly elastic collisions; i.e. the kinetic energy of the molecules is
conserved in all collisions, but may be transferred between them.
The speed of molecules in gases
Although the third premise means that the mean molecular energy is constant at constant
temperature, the energies, and hence the velocities of the molecules, will be distributed
over a wide range. The distribution of molecular speeds follows the Maxwell
distribution of speeds. Mathematically, the distribution is given by:
where f(s)ds is the probability of a molecule having a velocity in the range from s to s+ds,
N is the number and M is the molar mass of the gaseous molecules. At low temperatures,