Page 20 - Instant notes
P. 20
A2
MOLECULAR BEHAVIOR IN PERFECT
GASES
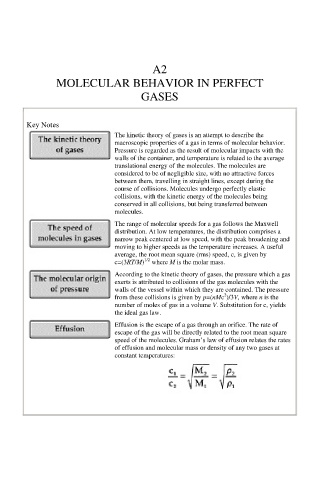
Key Notes
The kinetic theory of gases is an attempt to describe the
macroscopic properties of a gas in terms of molecular behavior.
Pressure is regarded as the result of molecular impacts with the
walls of the container, and temperature is related to the average
translational energy of the molecules. The molecules are
considered to be of negligible size, with no attractive forces
between them, travelling in straight lines, except during the
course of collisions. Molecules undergo perfectly elastic
collisions, with the kinetic energy of the molecules being
conserved in all collisions, but being transferred between
molecules.
The range of molecular speeds for a gas follows the Maxwell
distribution. At low temperatures, the distribution comprises a
narrow peak centered at low speed, with the peak broadening and
moving to higher speeds as the temperature increases. A useful
average, the root mean square (rms) speed, c, is given by
1/2
c=(3RT/M) where M is the molar mass.
According to the kinetic theory of gases, the pressure which a gas
exerts is attributed to collisions of the gas molecules with the
walls of the vessel within which they are contained. The pressure
2
from these collisions is given by p=(nMc )/3V, where n is the
number of moles of gas in a volume V. Substitution for c, yields
the ideal gas law.
Effusion is the escape of a gas through an orifice. The rate of
escape of the gas will be directly related to the root mean square
speed of the molecules. Graham’s law of effusion relates the rates
of effusion and molecular mass or density of any two gases at
constant temperatures: