Page 18 - Instant notes
P. 18
Physical Chemistry 4
A, p B for component B, etc. With this definition, it follows from the perfect gas equation
that the partial pressure for each component is given by:
p x=n xR T/V
where p x is the partial pressure of n x moles of component x.
The total pressure exerted by a mixture of ideal gases is related to the partial pressures
through Dalton’s law, which may be stated as,
‘the total pressure exerted by a mixture of ideal gases in a volume is equal to the
arithmetic sum of the partial pressures’.
If a gas mixture is comprised of, for example, n A, n B, and n C moles of three ideal gases,
A, B, and C, then the total pressure is given by:
P total=p A+p B+p C=n AR T/V+n BR T/V+n CR T/V=(n A+ n B+ n C)R T/V
=n totalR T/V
where n total is the total number of moles of gas, making this a simple restatement of the
ideal gas law.
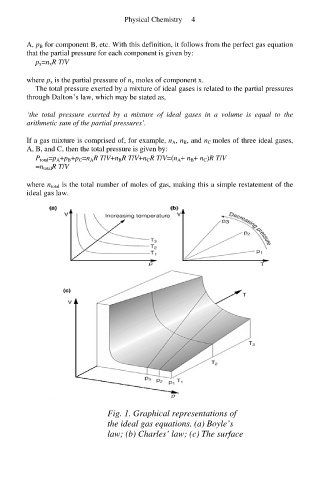
Fig. 1. Graphical representations of
the ideal gas equations. (a) Boyle’s
law; (b) Charles’ law; (c) The surface