Page 290 - Instant notes
P. 290
Physical chemistry 276
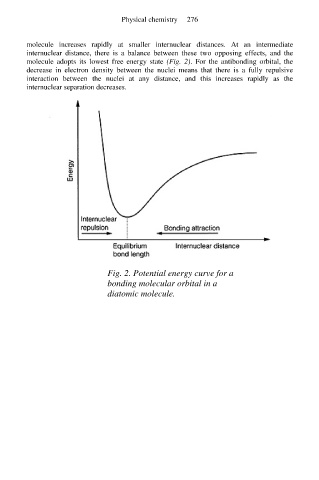
molecule increases rapidly at smaller internuclear distances. At an intermediate
internuclear distance, there is a balance between these two opposing effects, and the
molecule adopts its lowest free energy state (Fig. 2). For the antibonding orbital, the
decrease in electron density between the nuclei means that there is a fully repulsive
interaction between the nuclei at any distance, and this increases rapidly as the
internuclear separation decreases.
Fig. 2. Potential energy curve for a
bonding molecular orbital in a
diatomic molecule.