Page 293 - Instant notes
P. 293
Molecular orbital theory of diatomic molecules II 279
electrons are placed in pairs into the molecular orbitals, with the lowest energy molecular
orbitals being filled first.
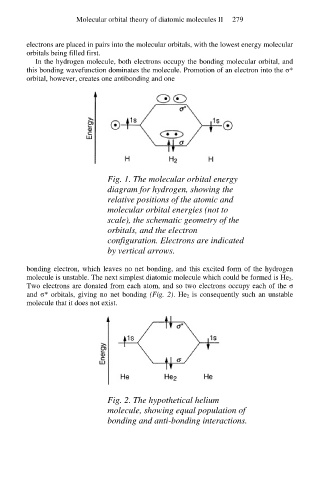
In the hydrogen molecule, both electrons occupy the bonding molecular orbital, and
this bonding wavefunction dominates the molecule. Promotion of an electron into the σ*
orbital, however, creates one antibonding and one
Fig. 1. The molecular orbital energy
diagram for hydrogen, showing the
relative positions of the atomic and
molecular orbital energies (not to
scale), the schematic geometry of the
orbitals, and the electron
configuration. Electrons are indicated
by vertical arrows.
bonding electron, which leaves no net bonding, and this excited form of the hydrogen
molecule is unstable. The next simplest diatomic molecule which could be formed is He 2.
Two electrons are donated from each atom, and so two electrons occupy each of the σ
and σ* orbitals, giving no net bonding (Fig. 2). He 2 is consequently such an unstable
molecule that it does not exist.
Fig. 2. The hypothetical helium
molecule, showing equal population of
bonding and anti-bonding interactions.