Page 296 - Instant notes
P. 296
Physical chemistry 282
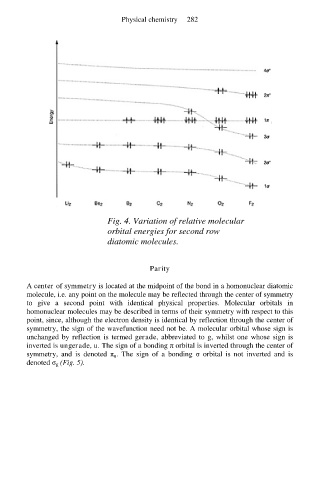
Fig. 4. Variation of relative molecular
orbital energies for second row
diatomic molecules.
Parity
A center of symmetry is located at the midpoint of the bond in a homonuclear diatomic
molecule, i.e. any point on the molecule may be reflected through the center of symmetry
to give a second point with identical physical properties. Molecular orbitals in
homonuclear molecules may be described in terms of their symmetry with respect to this
point, since, although the electron density is identical by reflection through the center of
symmetry, the sign of the wavefunction need not be. A molecular orbital whose sign is
unchanged by reflection is termed gerade, abbreviated to g, whilst one whose sign is
inverted is ungerade, u. The sign of a bonding π orbital is inverted through the center of
symmetry, and is denoted π u. The sign of a bonding σ orbital is not inverted and is
denoted σ g (Fig. 5).