Page 300 - Instant notes
P. 300
Physical chemistry 286
Dipole moments
A polar covalent bond implies that one atom in a molecule will be more positively
charged than the other. The positive charge, +q and the negative charge, −q, separated by
a distance R, give rise to an electric dipole moment, µ. This is a vector directed from the
positive to the negative charge across the molecule, with magnitude qR. This vector is
usually represented by an arrow directed from the positive to the negative charge, with a
positive sign included to indicate the positive end, thus: .
In order to generate convenient values, the dipole moment is generally reported in
debye, D, where 1 debye is equal to 3.336×10 −30 C m. Water, for example, has a dipole
moment of 1.85 D. The size of the dipole moment in debyes between two atoms, A and B
may often be estimated from their respective Pauling electronegativities, χ(A) and χ(B):
µ≈χ(A)−χ(B)
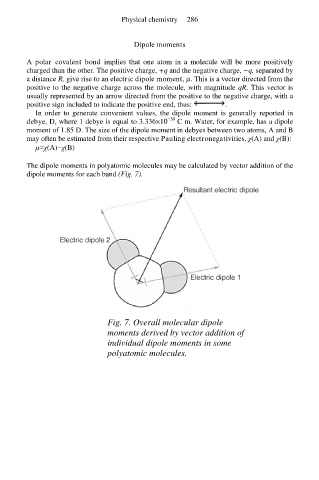
The dipole moments in polyatomic molecules may be calculated by vector addition of the
dipole moments for each band (Fig. 7).
Fig. 7. Overall molecular dipole
moments derived by vector addition of
individual dipole moments in some
polyatomic molecules.