Page 299 - Instant notes
P. 299
Molecular orbital theory of diatomic molecules II 285
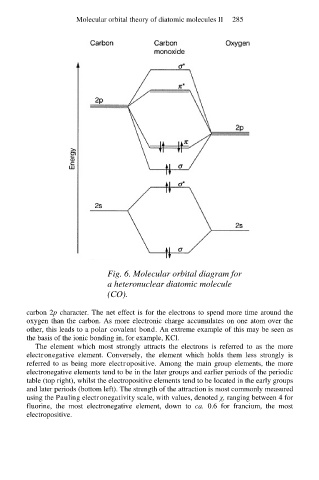
Fig. 6. Molecular orbital diagram for
a heteronuclear diatomic molecule
(CO).
carbon 2p character. The net effect is for the electrons to spend more time around the
oxygen than the carbon. As more electronic charge accumulates on one atom over the
other, this leads to a polar covalent bond. An extreme example of this may be seen as
the basis of the ionic bonding in, for example, KCl.
The element which most strongly attracts the electrons is referred to as the more
electronegative element. Conversely, the element which holds them less strongly is
referred to as being more electropositive. Among the main group elements, the more
electronegative elements tend to be in the later groups and earlier periods of the periodic
table (top right), whilst the electropositive elements tend to be located in the early groups
and later periods (bottom left). The strength of the attraction is most commonly measured
using the Pauling electronegativity scale, with values, denoted χ, ranging between 4 for
fluorine, the most electronegative element, down to ca. 0.6 for francium, the most
electropositive.