Page 314 - Instant notes
P. 314
Physical chemistry 300
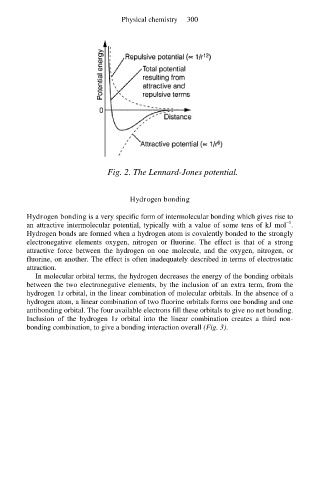
Fig. 2. The Lennard-Jones potential.
Hydrogen bonding
Hydrogen bonding is a very specific form of intermolecular bonding which gives rise to
−1
an attractive intermolecular potential, typically with a value of some tens of kJ mol .
Hydrogen bonds are formed when a hydrogen atom is covalently bonded to the strongly
electronegative elements oxygen, nitrogen or fluorine. The effect is that of a strong
attractive force between the hydrogen on one molecule, and the oxygen, nitrogen, or
fluorine, on another. The effect is often inadequately described in terms of electrostatic
attraction.
In molecular orbital terms, the hydrogen decreases the energy of the bonding orbitals
between the two electronegative elements, by the inclusion of an extra term, from the
hydrogen 1s orbital, in the linear combination of molecular orbitals. In the absence of a
hydrogen atom, a linear combination of two fluorine orbitals forms one bonding and one
antibonding orbital. The four available electrons fill these orbitals to give no net bonding.
Inclusion of the hydrogen 1s orbital into the linear combination creates a third non-
bonding combination, to give a bonding interaction overall (Fig. 3).