Page 309 - Instant notes
P. 309
H6
WEAK INTERMOLECULAR
INTERACTIONS
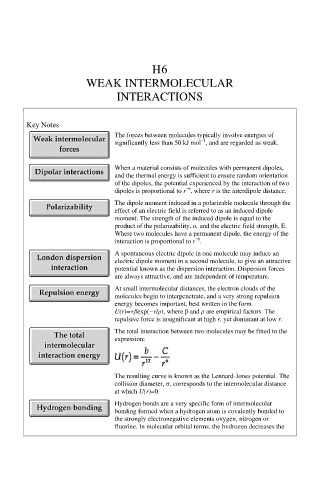
Key Notes
The forces between molecules typically involve energies of
−1
significantly less than 50 kJ mol , and are regarded as weak.
When a material consists of molecules with permanent dipoles,
and the thermal energy is sufficient to ensure random orientation
of the dipoles, the potential experienced by the interaction of two
−6
dipoles is proportional to r , where r is the interdipole distance.
The dipole moment induced in a polarizable molecule through the
effect of an electric field is referred to as an induced dipole
moment. The strength of the induced dipole is equal to the
product of the polarizability, α, and the electric field strength, E.
Where two molecules have a permanent dipole, the energy of the
−6
interaction is proportional to r .
A spontaneous electric dipole in one molecule may induce an
electric dipole moment in a second molecule, to give an attractive
potential known as the dispersion interaction. Dispersion forces
are always attractive, and are independent of temperature.
At small intermolecular distances, the electron clouds of the
molecules begin to interpenetrate, and a very strong repulsion
energy becomes important, best written in the form
U(r)=+βexp(−r/ρ), where β and ρ are empirical factors. The
repulsive force is insignificant at high r, yet dominant at low r.
The total interaction between two molecules may be fitted to the
expression:
The resulting curve is known as the Lennard-Jones potential. The
collision diameter, σ, corresponds to the intermolecular distance
at which U(r)=0.
Hydrogen bonds are a very specific form of intermolecular
bonding formed when a hydrogen atom is covalently bonded to
the strongly electronegative elements oxygen, nitrogen or
fluorine. In molecular orbital terms, the hydrogen decreases the