Page 306 - Instant notes
P. 306
Physical chemistry 292
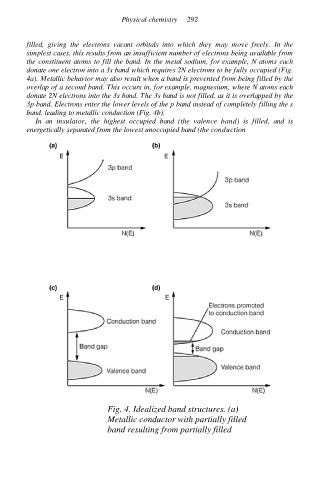
filled, giving the electrons vacant orbitals into which they may move freely. In the
simplest cases, this results from an insufficient number of electrons being available from
the constituent atoms to fill the band. In the metal sodium, for example, N atoms each
donate one electron into a 3s band which requires 2N electrons to be fully occupied (Fig.
4a). Metallic behavior may also result when a band is prevented from being filled by the
overlap of a second band. This occurs in, for example, magnesium, where N atoms each
donate 2N electrons into the 3s band. The 3s band is not filled, as it is overlapped by the
3p band. Electrons enter the lower levels of the p band instead of completely filling the s
band, leading to metallic conduction (Fig. 4b).
In an insulator, the highest occupied band (the valence band) is filled, and is
energetically separated from the lowest unoccupied band (the conduction
Fig. 4. Idealized band structures. (a)
Metallic conductor with partially filled
band resulting from partially filled