Page 37 - Instant notes
P. 37
Crystalline solids 23
crystalline materials, which typically have highly regular forms with flat crystal faces. It
is this order and regularity which enables much simpler structural studies of crystalline
materials.
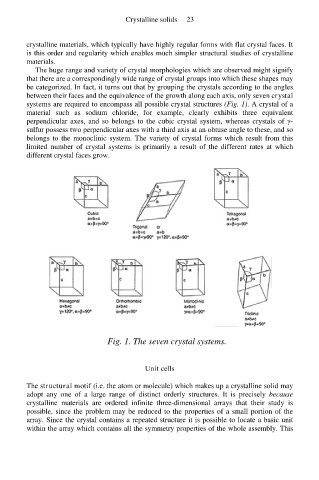
The huge range and variety of crystal morphologies which are observed might signify
that there are a correspondingly wide range of crystal groups into which these shapes may
be categorized. In fact, it turns out that by grouping the crystals according to the angles
between their faces and the equivalence of the growth along each axis, only seven crystal
systems are required to encompass all possible crystal structures (Fig. 1). A crystal of a
material such as sodium chloride, for example, clearly exhibits three equivalent
perpendicular axes, and so belongs to the cubic crystal system, whereas crystals of γ-
sulfur possess two perpendicular axes with a third axis at an obtuse angle to these, and so
belongs to the monoclinic system. The variety of crystal forms which result from this
limited number of crystal systems is primarily a result of the different rates at which
different crystal faces grow.
Fig. 1. The seven crystal systems.
Unit cells
The structural motif (i.e. the atom or molecule) which makes up a crystalline solid may
adopt any one of a large range of distinct orderly structures. It is precisely because
crystalline materials are ordered infinite three-dimensional arrays that their study is
possible, since the problem may be reduced to the properties of a small portion of the
array. Since the crystal contains a repeated structure it is possible to locate a basic unit
within the array which contains all the symmetry properties of the whole assembly. This