Page 39 - Instant notes
P. 39
Crystalline solids 25
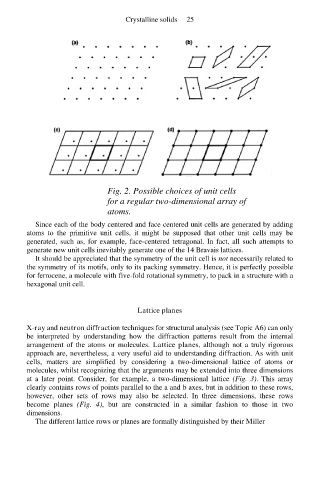
Fig. 2. Possible choices of unit cells
for a regular two-dimensional array of
atoms.
Since each of the body centered and face centered unit cells are generated by adding
atoms to the primitive unit cells, it might be supposed that other unit cells may be
generated, such as, for example, face-centered tetragonal. In fact, all such attempts to
generate new unit cells inevitably generate one of the 14 Bravais lattices.
It should be appreciated that the symmetry of the unit cell is not necessarily related to
the symmetry of its motifs, only to its packing symmetry. Hence, it is perfectly possible
for ferrocene, a molecule with five-fold rotational symmetry, to pack in a structure with a
hexagonal unit cell.
Lattice planes
X-ray and neutron diffraction techniques for structural analysis (see Topic A6) can only
be interpreted by understanding how the diffraction patterns result from the internal
arrangement of the atoms or molecules. Lattice planes, although not a truly rigorous
approach are, nevertheless, a very useful aid to understanding diffraction. As with unit
cells, matters are simplified by considering a two-dimensional lattice of atoms or
molecules, whilst recognizing that the arguments may be extended into three dimensions
at a later point. Consider, for example, a two-dimensional lattice (Fig. 3). This array
clearly contains rows of points parallel to the a and b axes, but in addition to these rows,
however, other sets of rows may also be selected. In three dimensions, these rows
become planes (Fig. 4), but are constructed in a similar fashion to those in two
dimensions.
The different lattice rows or planes are formally distinguished by their Miller