Page 81 - Instant notes
P. 81
Fundamentals of equilibria 67
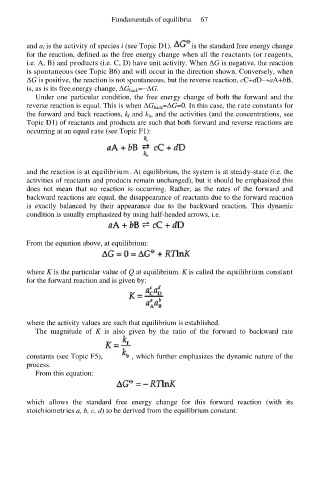
and a i is the activity of species i (see Topic D1). is the standard free energy change
for the reaction, defined as the free energy change when all the reactants (or reagents,
i.e. A, B) and products (i.e. C, D) have unit activity. When ∆G is negative, the reaction
is spontaneous (see Topic B6) and will occur in the direction shown. Conversely, when
∆G is positive, the reaction is not spontaneous, but the reverse reaction, cC+dD→aA+bB,
is, as is its free energy change, ∆G back=−∆G.
Under one particular condition, the free energy change of both the forward and the
reverse reaction is equal. This is when ∆G back=∆G=0. In this case, the rate constants for
the forward and back reactions, k f and k b, and the activities (and the concentrations, see
Topic D1) of reactants and products are such that both forward and reverse reactions are
occurring at an equal rate (see Topic F1):
and the reaction is at equilibrium. At equilibrium, the system is at steady-state (i.e. the
activities of reactants and products remain unchanged), but it should be emphasized this
does not mean that no reaction is occurring. Rather, as the rates of the forward and
backward reactions are equal, the disappearance of reactants due to the forward reaction
is exactly balanced by their appearance due to the backward reaction. This dynamic
condition is usually emphasized by using half-headed arrows, i.e.
From the equation above, at equilibrium:
where K is the particular value of Q at equilibrium. K is called the equilibrium constant
for the forward reaction and is given by:
where the activity values are such that equilibrium is established.
The magnitude of K is also given by the ratio of the forward to backward rate
constants (see Topic F5), , which further emphasizes the dynamic nature of the
process.
From this equation:
which allows the standard free energy change for this forward reaction (with its
stoichiometries a, b, c, d) to be derived from the equilibrium constant.