Page 85 - Instant notes
P. 85
Fundamentals of equilibria 71
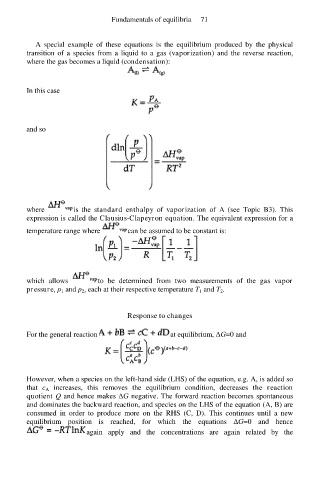
A special example of these equations is the equilibrium produced by the physical
transition of a species from a liquid to a gas (vaporization) and the reverse reaction,
where the gas becomes a liquid (condensation):
In this case
and so
where is the standard enthalpy of vaporization of A (see Topic B3). This
expression is called the Clausius-Clapeyron equation. The equivalent expression for a
temperature range where can be assumed to be constant is:
which allows to be determined from two measurements of the gas vapor
pressure, p 1 and p 2, each at their respective temperature T 1 and T 2.
Response to changes
For the general reaction at equilibrium, ∆G=0 and
However, when a species on the left-hand side (LHS) of the equation, e.g. A, is added so
that c A increases, this removes the equilibrium condition, decreases the reaction
quotient Q and hence makes ∆G negative. The forward reaction becomes spontaneous
and dominates the backward reaction, and species on the LHS of the equation (A, B) are
consumed in order to produce more on the RHS (C, D). This continues until a new
equilibrium position is reached, for which the equations ∆G=0 and hence
again apply and the concentrations are again related by the