Page 102 - Introduction to Colloid and Surface Chemistry
P. 102
92 Liquid-gas and liquid-liquid interfaces
The alternative approach is to treat micellisation as a simple phase
separation of surfactant in an associated form, with the unassociated
surfactant concentration remaining practically constant above the
c.m.c.
In the build-up from surfactant monomers to micelles, the
existence, albeit transitorily, of intermediate levels of aggregation is
to be expected. Close examination of experimental evidence suggests
that there may be some smoothness of property change at the c.m.c. ,
but that sub-micelle aggregates exist only in trace amounts,
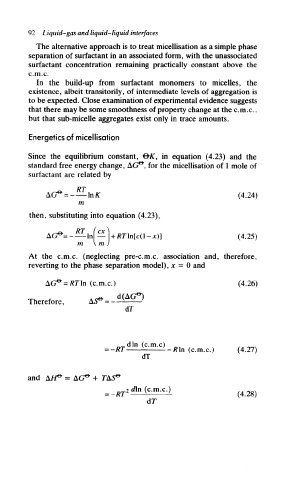
Energetics of micellisation
Since the equilibrium constant, &K, in equation (4.23) and the
standard free energy change, AG^, for the micellisation of 1 mole of
surfactant are related by
RT
AG° = -- InK (4.24)
m
then, substituting into equation (4.23),
RT ( ex ^\
In — \ + RT\n(c(l-x)] (4.25)
V m )
At the c.m.c. (neglecting pre-c.m.c. association and, therefore,
reverting to the phase separation model), x - 0 and
(c.m.c.) (4.26)
Therefore, *S» =
dF
dT
and
(c.m.c.)