Page 105 - Introduction to Colloid and Surface Chemistry
P. 105
Liquid-gas and liquid-liquid interfaces 95
AW
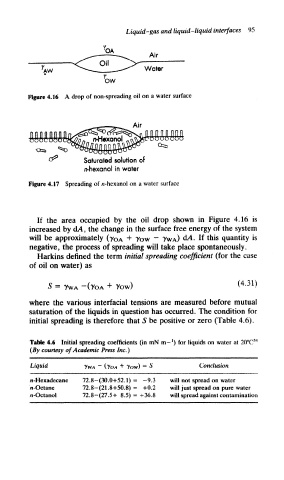
Figure 4.16 A drop of non-spreading oil on a water surface
^ Saturated solution of
n-hexanol in water
Figure 4.17 Spreading of n-hexanol on a water surface
If the area occupied by the oil drop shown in Figure 4.16 is
increased by d/t, the change in the surface free energy of the system
will be approximately (-y 0A + 7ow ~ ?WA) d/i. If this quantity is
negative, the process of spreading will take place spontaneously.
Harkins defined the term initial spreading coefficient (for the case
of oil on water) as
S = ~OxoA + Tow) (4.31)
where the various interfacial tensions are measured before mutual
saturation of the liquids in question has occurred. The condition for
initial spreading is therefore that 5 be positive or zero (Table 4.6).
1
Table 4.6 Initial spreading coefficients (in mN m- ) for liquids on water at 20°C 54
(By courtesy of Academic Press Inc.)
Liquid 7wA - (JOA + 7ow) = S Conclusion
rt-Hexadecane 72.8-(30.0+52.1) = -9.3 will not spread on water
H-Octane 72.8-(21. 8+50.8) = +0.2 will just spread on pure water
H-Octanol 72.8-(27.5+ 8.5) = +36.8 will spread against contamination