Page 150 - Introduction to Colloid and Surface Chemistry
P. 150
140 The solid-gas interface
gradually replaced by a peak at 283 eV on heating to 350 K as a result
73
of surface dissociation . Likewise, chemical shifts can be exploited
to study the oxidation states of surface atoms.
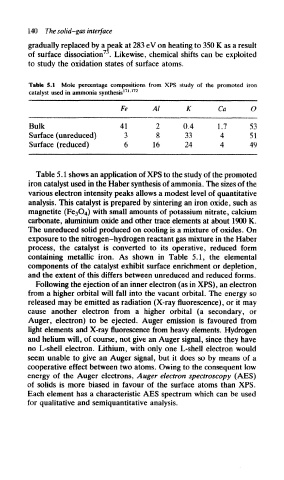
Table 5.1 Mole percentage compositions from XPS study of the promoted iron
171 172
catalyst used in ammonia synthesis '
Fe Al K Ca O
Bulk 41 2 0.4 1.7 53
Surface (unreduced) 3 8 33 4 51
Surface (reduced) 6 16 24 4 49
Table 5.1 shows an application of XPS to the study of the promoted
iron catalyst used in the Haber synthesis of ammonia. The sizes of the
various electron intensity peaks allows a modest level of quantitative
analysis. This catalyst is prepared by sintering an iron oxide, such as
magnetite (Fe 3O 4) with small amounts of potassium nitrate, calcium
carbonate, aluminium oxide and other trace elements at about 1900 K.
The unreduced solid produced on cooling is a mixture of oxides. On
exposure to the nitrogen-hydrogen reactant gas mixture in the Haber
process, the catalyst is converted to its operative, reduced form
containing metallic iron. As shown in Table 5.1, the elemental
components of the catalyst exhibit surface enrichment or depletion,
and the extent of this differs between unreduced and reduced forms.
Following the ejection of an inner electron (as in XPS), an electron
from a higher orbital will fall into the vacant orbital. The energy so
released may be emitted as radiation (X-ray fluorescence), or it may
cause another electron from a higher orbital (a secondary, or
Auger, electron) to be ejected. Auger emission is favoured from
light elements and X-ray fluorescence from heavy elements. Hydrogen
and helium will, of course, not give an Auger signal, since they have
no L-shell electron. Lithium, with only one L-shell electron would
seem unable to give an Auger signal, but it does so by means of a
cooperative effect between two atoms. Owing to the consequent low
energy of the Auger electrons, Auger electron spectroscopy (AES)
of solids is more biased in favour of the surface atoms than XPS.
Each element has a characteristic AES spectrum which can be used
for qualitative and semiquantitative analysis.