Page 217 - Introduction to Colloid and Surface Chemistry
P. 217
206 Charged interfaces
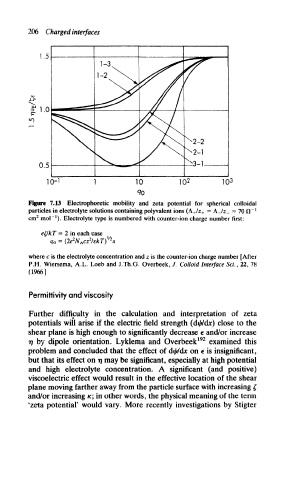
Figure 7.13 Electrophoretic mobility and zeta potential for spherical colloidal
particles in electrolyte solutions containing polyvalent ions (A. +/z + = A_/z_ = 70 ft~ !
2
cm mol""'). Electrolyte type is numbered with counter-ion charge number first:
eg/kT — 2 in each case
V2
2
2
go = (2e N Acz /EkT) a
where c is the electrolyte concentration and z is the counter-ion charge number [After
P,H. Wiersema, A.L. Loeb and J.Th.G. Overbeek, /. Colloid Interface ScL, 22, 78
(1966]
Permittivity and viscosity
Further difficulty in the calculation and interpretation of zeta
potentials will arise if the electric field strength (d^d*) close to the
shear plane is high enough to significantly decrease e and/or increase
192
17 by dipole orientation. Lyklema and Overbeek examined this
problem and concluded that the effect of dift/dx on e is insignificant,
but that its effect on 17 may be significant, especially at high potential
and high electrolyte concentration. A significant (and positive)
viscoelectric effect would result in the effective location of the shear
plane moving farther away from the particle surface with increasing £
and/or increasing K; in other words, the physical meaning of the term
'zeta potential' would vary. More recently investigations by Stigter