Page 222 - Introduction to Colloid and Surface Chemistry
P. 222
Colloid stability 211
3
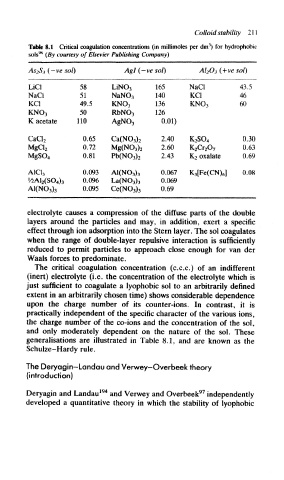
Table 8.1 Critical coagulation concentrations (in millimoles per dm ) for hydrophobic
96
sols (By courtesy of Elsevier Publishing Company)
As2$3 (~ve so/) Agl (—ve sol) A1 2O 3 (+ve sol)
LiCl 58 UNO, 165 NaCl 43.5
NaCl 51 NaNO 3 140 KCI 46
KCI 49.5 KNO, 136 KNO 3 60
50 RbNO, 126
KNO 3
K acetate 110 AgN0 3 0.01)
0.65 2.40 0.30
CaCI 2 Ca(N0 3) 2 K 2SO 4
0.72 2.60 0.63
MgCI 2 Mg(N0 3) 2 K 2Cr 20 7
0.81 2.43 K 2 oxalate 0.69
MgSO 4 Pb(N0 3) 2
0.093 0.067 K 3[Fe(CN) 6] 0.08
A1C1 3 A1(N0 3) 3
0.096 0.069
M>A1 2(SO 4) 3 La(N0 3) 3
0.095 0.69
AI(N0 3) 3 Ce(N0 3) 3
electrolyte causes a compression of the diffuse parts of the double
layers around the particles and may, in addition, exert a specific
effect through ion adsorption into the Stern layer. The sol coagulates
when the range of double-layer repulsive interaction is sufficiently
reduced to permit particles to approach close enough for van der
Waals forces to predominate.
The critical coagulation concentration (c.c.c.) of an indifferent
(inert) electrolyte (i.e. the concentration of the electrolyte which is
just sufficient to coagulate a lyophobic sol to an arbitrarily defined
extent in an arbitrarily chosen time) shows considerable dependence
upon the charge number of its counter-ions. In contrast, it is
practically independent of the specific character of the various ions,
the charge number of the co-ions and the concentration of the sol,
and only moderately dependent on the nature of the sol. These
generalisations are illustrated in Table 8.1, and are known as the
Schulze-Hardy rule.
The Deryagin-Landau and Verwey-Overbeek theory
(introduction)
97
Deryagin and Landau 194 and Verwey and Overbeek independently
developed a quantitative theory in which the stability of lyophobic