Page 225 - Introduction to Colloid and Surface Chemistry
P. 225
214 Colloid stability
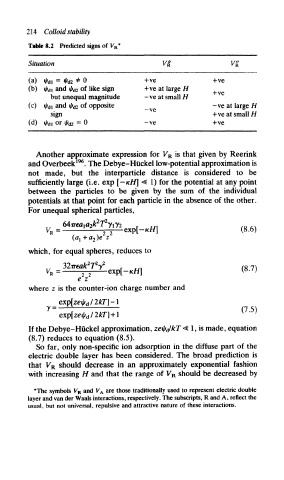
Tabk 8.2 Predicted signs of V R*
Situation
(a) fai = ^r d; 2 * 0 H-ve +ve
(b) tjf di and 4> d2 of like sign +ve at large H
but unequal magnitude -ve at small//
(c) tj/ di and t/!r d2 of opposite — ve -ve at large H
sign +ve at small H
(d) if) Al or ^ d 2 = 0 — ve +ve
Another approximate expression for V R is that given by Reerink
196
and Overbeek . The Debye-Hiickel low-potential approximation is
not made, but the interparticle distance is considered to be
sufficiently large (i.e. exp [— K//] < 1) for the potential at any point
between the particles to be given by the sum of the individual
potentials at that point for each particle in the absence of the other.
For unequal spherical particles,
2 2
? z
which, for equal spheres, reduces to
e z
where 2 is the counter-ion charge number and
If the Debye-Hiickel approximation, zetf/ d/kT<3 1, is made, equation
(8.7) reduces to equation (8.5).
So far, only non-specific ion adsorption in the diffuse part of the
electric double layer has been considered. The broad prediction is
that V R should decrease in an approximately exponential fashion
with increasing H and that the range of V R should be decreased by
*The symbols V R and V A are those traditionally used to represent electric double
layer and van der Waals interactions, respectively. The subscripts, R and A, reflect the
usual, but not universal, repulsive and attractive nature of these interactions,