Page 298 - Introduction to Colloid and Surface Chemistry
P. 298
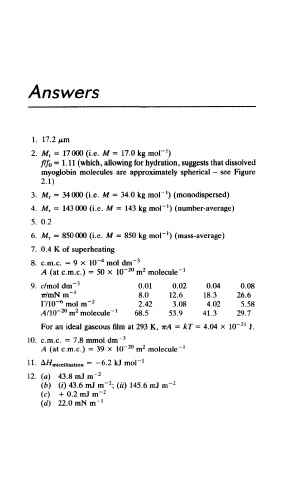
Answers
1. 17.2 /urn
1
2. M r = 17000 (i.e. M = 17.0 kg mol" )
/// 0 =1.11 (which, allowing for hydration, suggests that dissolved
myoglobin molecules are approximately spherical - see Figure
2.1)
1
3. M r = 34000 (i.e. M = 34.0 kg mol" ) (monodispersed)
1
4. M r = 143000 (i.e. M = 143 kg inoP ) (number-average)
5. 0.2
1
6. M r = 850000 (i.e. M = 850 kg moP ) (mass-average)
7. 0.4 K of superheating
4
8. c.m.c. = 9 x 10~ mol dm" 3
2
20
A (at c.m.c.) = 50 x 10~ m molecule' 1
9. c/mol dm" 3 0.01 0.02 0.04 0.08
ir/mN m" 1 8.0 12.6 18.3 26.6
6
F/10~ mol m~ 2 2.42 3.08 4.02 5.58
20 2 1
A/lQ- m molecule" 68.5 53.9 41.3 29.7
21
For an ideal gaseous film at 293 K, irA = kT = 4.04 x 10~ J.
10. c.m.c. = 7.8 mmol dm" 3
20
2
A (at c.m.c.) = 39 x 10~ m molecule" 1
1
11. A/^ miceii isation = — 6.2 kJ moP
12. (a) 43.8 mJ m" 2
2
(b) (/) 43.6 mJ m" ; (#) 145.6 mJ m" 2
(c) -f 0.2 mJ m" 2
1
(d) 22.0 mNm"