Page 293 - Introduction to Colloid and Surface Chemistry
P. 293
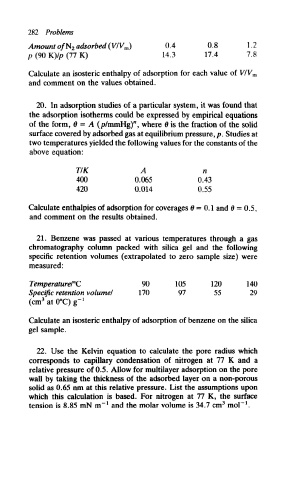
282 Problems
Amount of'N 2 adsorbed (VIV m) 0.4 0.8 1.2
p (90 K)/p (77 K) 14.3 17.4 7.8
Calculate an isosteric enthalpy of adsorption for each value of VIV m
and comment on the values obtained.
20. In adsorption studies of a particular system, it was found that
the adsorption isotherms could be expressed by empirical equations
of the form, 6 = A (p/mmHg)", where 9 is the fraction of the solid
surface covered by adsorbed gas at equilibrium pressure, p. Studies at
two temperatures yielded the following values for the constants of the
above equation:
TIK A n
400 0.065 0.43
420 0.014 0.55
Calculate enthalpies of adsorption for coverages 0 = 0.1 and & = 0,5,
and comment on the results obtained.
21. Benzene was passed at various temperatures through a gas
chromatography column packed with silica gel and the following
specific retention volumes (extrapolated to zero sample size) were
measured:
TemperaturerC 90 105 120 140
Specific retention volume! 170 97 55 29
3
(cm at 0°C) g- 1
Calculate an isosteric enthalpy of adsorption of benzene on the silica
gel sample.
22. Use the Kelvin equation to calculate the pore radius which
corresponds to capillary condensation of nitrogen at 77 K and a
relative pressure of 0.5. Allow for multilayer adsorption on the pore
wall by taking the thickness of the adsorbed layer on a non-porous
solid as 0.65 nm at this relative pressure. List the assumptions upon
which this calculation is based. For nitrogen at 77 K, the surface
3
1
1
tension is 8.85 mN m" and the molar volume is 34.7 cm mol" .