Page 289 - Introduction to Colloid and Surface Chemistry
P. 289
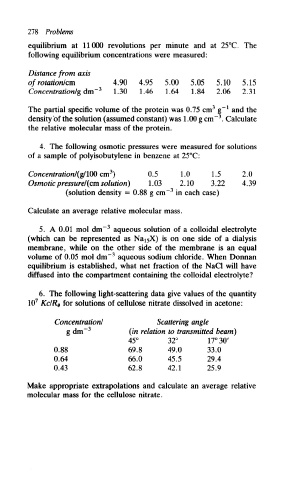
278 Problems
equilibrium at 11000 revolutions per minute and at 25°C. The
following equilibrium concentrations were measured:
Distance from axis
of rotation/cm 4.90 4.95 5.00 5.05 5.10 5.15
Concentration/'g dm~ 3 1.30 1.46 1.64 1.84 2.06 2.3!
3
1
The partial specific volume of the protein was 0.75 cm g" and the
3
density of the solution (assumed constant) was 1.00 g cm" . Calculate
the relative molecular mass of the protein.
4. The following osmotic pressures were measured for solutions
of a sample of polyisobutylene in benzene at 25°C:
3
Concentration/(g/m cm ) 0.5 1.0 1.5 2.0
Osmotic pressure/'(cm solution) 1.03 2.10 3.22 4.39
3
(solution density = 0.88 g cm~ in each case)
Calculate an average relative molecular mass.
3
5. A 0.01 mol dm~ aqueous solution of a colloidal electrolyte
(which can be represented as Na^X) is on one side of a dialysis
membrane, while on the other side of the membrane is an equal
3
volume of 0.05 mol dm" aqueous sodium chloride. When Donnan
equilibrium is established, what net fraction of the NaCl will have
diffused into the compartment containing the colloidal electrolyte?
6. The following light-scattering data give values of the quantity
10 7 Kc/Re for solutions of cellulose nitrate dissolved in acetone:
Concentration/ Scattering angle
g dm~ 3 (in relation to transmitted beam)
45° 32° 17° 30'
0.88 69.8 49.0 33.0
0.64 66.0 45.5 29.4
0.43 62.8 42.1 25.9
Make appropriate extrapolations and calculate an average relative
molecular mass for the cellulose nitrate.