Page 291 - Introduction to Colloid and Surface Chemistry
P. 291
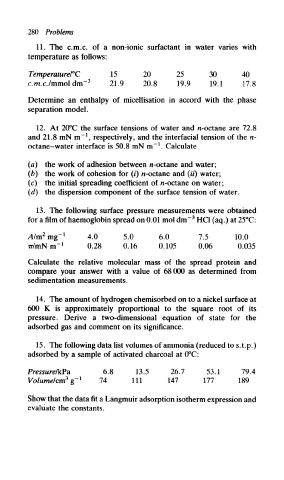
280 Problems
11. The c.m.c. of a non-ionic surfactant in water varies with
temperature as follows:
Temperature/°C 15 20 25 30 40
3
c.m.c./mmol dm" 21.9 20.8 19.9 19.1 17.8
Determine an enthalpy of micellisation in accord with the phase
separation model.
12. At 20°C the surface tensions of water and n-octane are 72.8
1
and 21.8 mN m" , respectively, and the interfacial tension of the n-
1
octane-water interface is 50.8 mN m" . Calculate
(a) the work of adhesion between n-octane and water;
(b) the work of cohesion for (i) n-octane and (M) water;
(c) the initial spreading coefficient of n-octane on water;
(d) the dispersion component of the surface tension of water.
13. The following surface pressure measurements were obtained
3
for a film of haemoglobin spread on 0.01 mol dm~ HC1 (aq.) at 25°C:
2
A/m mg- 1 4.0 5.0 6.0 7.5 10.0
Tr/mNm" 1 0.28 0.16 0.105 0.06 0.035
Calculate the relative molecular mass of the spread protein and
compare your answer with a value of 68000 as determined from
sedimentation measurements.
14. The amount of hydrogen chemisorbed on to a nickel surface at
600 K is approximately proportional to the square root of its
pressure. Derive a two-dimensional equation of state for the
adsorbed gas and comment on its significance.
15. The following data list volumes of ammonia (reduced to s.t.p.)
adsorbed by a sample of activated charcoal at 0°C:
Pressure/kPa 6.8 13.5 26.7 53.1 79.4
3 1
Volume/cm g" 74 111 147 177 189
Show that the data fit a Langmuir adsorption isotherm expression and
evaluate the constants.