Page 74 - Introduction to Colloid and Surface Chemistry
P. 74
4 Liquid—gas and liquid-
liquid interfaces
Surface and interfacial tensions
It is well known that short-range forces of attraction exist between
molecules (see page 215), and are responsible for the existence of the
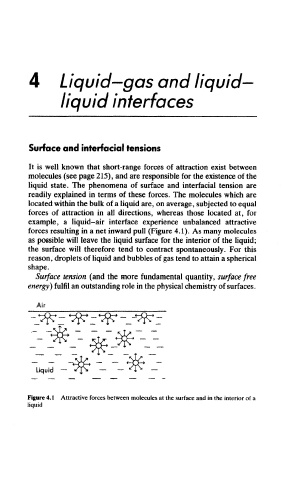
liquid state. The phenomena of surface and interfacial tension are
readily explained in terms of these forces. The molecules which are
located within the bulk of a liquid are, on average, subjected to equal
forces of attraction in all directions, whereas those located at, for
example, a liquid-air interface experience unbalanced attractive
forces resulting in a net inward pull (Figure 4.1). As many molecules
as possible will leave the liquid surface for the interior of the liquid;
the surface will therefore tend to contract spontaneously. For this
reason, droplets of liquid and bubbles of gas tend to attain a spherical
shape.
Surface tension (and the more fundamental quantity, surface free
energy) fulfil an outstanding role in the physical chemistry of surfaces.
Air
Liquid
Figure 4.1 Attractive forces between molecules at the surface and in the interior of a
liquid