Page 76 - Introduction to Colloid and Surface Chemistry
P. 76
66 Liquid-gas and liquid-liquid interfaces
hydrogen bonding (as, for example, in water) and metal bonding (as,
for example, in mercury). The relatively high values of the surface
tensions of water and mercury (see Table 4.1) reflect the contributions
of hydrogen bonding and metal bonding, respectively.
These forces are not appreciably influenced by one another, and so
may be assumed to be additive. The surface tension of water may,
therefore, be considered as the sum of a dispersion force contribution,
yw» and a hydrogen bonding contribution, - i.e.
Tw = Tw + 7w (4.1)
Similarly, the surface tension of mercury is made up of dispersion and
metal bond contributions:
_ »,d (4.2)
In the case of hydrocarbons the surface tension is entirely the result
of the dispersion force contribution.
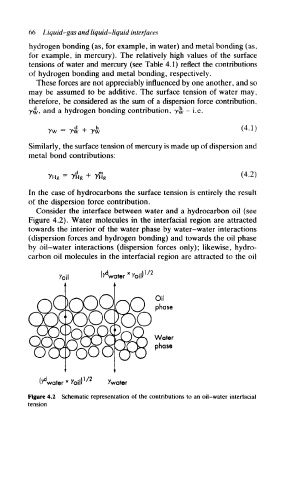
Consider the interface between water and a hydrocarbon oil (see
Figure 4.2). Water molecules in the interfacial region are attracted
towards the interior of the water phase by water-water interactions
(dispersion forces and hydrogen bonding) and towards the oil phase
by oil-water interactions (dispersion forces only); likewise, hydro-
carbon oil molecules in the interfacial region are attracted to the oil
Xoil) 1/2
phase
Water
phase
d
(X water*)'oil) 1/2
Figure 4.2 Schematic representation of the contributions to an oil-water interfacial
tension