Page 80 - Introduction to Colloid and Surface Chemistry
P. 80
70 Liquid-gas and liquid-liquid interfaces
Capillary rise method
This is, when properly performed, the most accurate method
available for determining surface tensions. Since the measurements
do not involve a disturbance of the surface, slow time effects can be
followed.
2r
z?zz
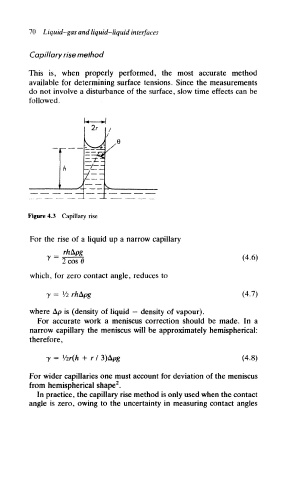
Figure 4,3 Capillary rise
For the rise of a liquid up a narrow capillary
(4.6)
2 cosO
which, for zero contact angle, reduces to
y = Vi rh&pg (4.7)
where Ap is (density of liquid — density of vapour).
For accurate work a meniscus correction should be made. In a
narrow capillary the meniscus will be approximately hemispherical:
therefore,
y = Vir(h + r I 3)Apg (4.8)
For wider capillaries one must account for deviation of the meniscus
2
from hemispherical shape .
In practice, the capillary rise method is only used when the contact
angle is zero, owing to the uncertainty in measuring contact angles