Page 37 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 37
26 Introduction to Transfer Phenomena in PEM Fuel Cells
The operating temperature is between 800°C and 1,000°C. At this
temperature, the ceramic has a very good ionic conductivity.
SOFCs are mainly found in stationary applications; however, some car
manufacturers see these fuel cells being used in the future because they
accept a wide variety of fuels. The power range of SOFC batteries is
between 1 kW and 10 MW [BLU 07].
1.2.1.6.1. Operating characteristics
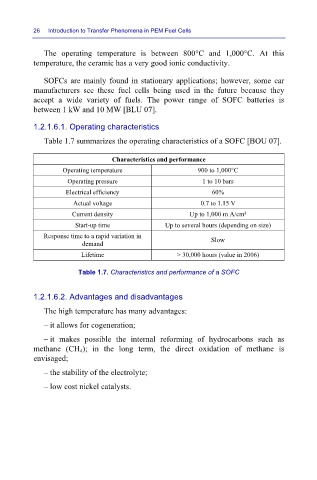
Table 1.7 summarizes the operating characteristics of a SOFC [BOU 07].
Characteristics and performance
Operating temperature 900 to 1,000°C
Operating pressure 1 to 10 bars
Electrical efficiency 60%
Actual voltage 0.7 to 1.15 V
Current density Up to 1,000 m A/cm²
Start-up time Up to several hours (depending on size)
Response time to a rapid variation in Slow
demand
Lifetime > 30,000 hours (value in 2006)
Table 1.7. Characteristics and performance of a SOFC
1.2.1.6.2. Advantages and disadvantages
The high temperature has many advantages:
– it allows for cogeneration;
– it makes possible the internal reforming of hydrocarbons such as
methane (CH 4); in the long term, the direct oxidation of methane is
envisaged;
– the stability of the electrolyte;
– low cost nickel catalysts.