Page 76 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 76
Charge Transfer Phenomena 65
2.3. The flow rates of reactants and products
There is a relationship between the current delivered by the cell, the flow
rate, the consumption of the reactants, and the production of water and heat.
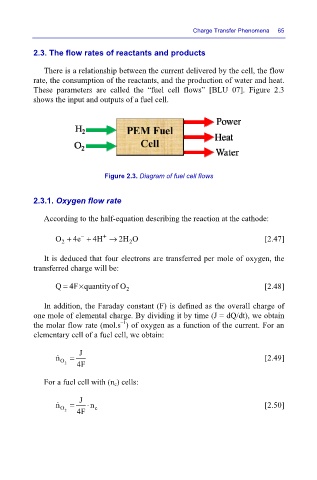
These parameters are called the “fuel cell flows” [BLU 07]. Figure 2.3
shows the input and outputs of a fuel cell.
Figure 2.3. Diagram of fuel cell flows
2.3.1. Oxygen flow rate
According to the half-equation describing the reaction at the cathode:
−
+
O + 4e + 4H → 2H O [2.47]
2
2
It is deduced that four electrons are transferred per mole of oxygen, the
transferred charge will be:
=
×
Q4F quantityof O 2 [2.48]
In addition, the Faraday constant (F) is defined as the overall charge of
one mole of elemental charge. By dividing it by time (J = dQ/dt), we obtain
–1
the molar flow rate (mol.s ) of oxygen as a function of the current. For an
elementary cell of a fuel cell, we obtain:
J
n O 2 = 4F [2.49]
For a fuel cell with (n c) cells:
J
n O 2 = 4F n ⋅ c [2.50]