Page 78 - Introduction to Transfer Phenomena in PEM Fuel Cells
P. 78
Charge Transfer Phenomena 67
–1
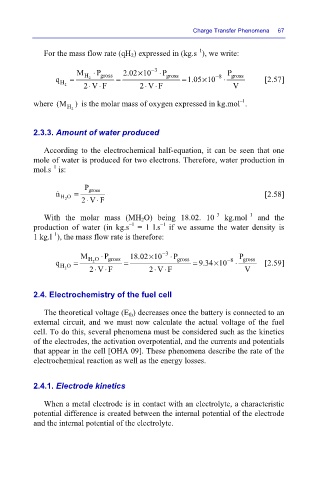
For the mass flow rate (qH 2) expressed in (kg.s ), we write:
P ⋅
×
gross
H
×
q H 2 = M 2V F = 2.02 10 − 3 ⋅ P ⋅ gross = 1.05 10 − 8 ⋅ P gross [2.57]
2
⋅
⋅
⋅
2V F
V
–1
where (M H 2 ) is the molar mass of oxygen expressed in kg.mol .
2.3.3. Amount of water produced
According to the electrochemical half-equation, it can be seen that one
mole of water is produced for two electrons. Therefore, water production in
–1
mol.s is:
P
n = gross [2.58]
2⋅ V F ⋅
H 2 O
–1
–3
With the molar mass (MH 2O) being 18.02. 10 kg.mol and the
–1
–1
production of water (in kg.s = 1 l.s if we assume the water density is
–1
1 kg.l ), the mass flow rate is therefore:
×
M HO P ⋅ gross 18.02 10 − 3 P ⋅ P
×
q HO = 2V F = 2V F gross = 9.34 10 − 8 ⋅ gross [2.59]
2
⋅
⋅
⋅
⋅
V
2
2.4. Electrochemistry of the fuel cell
The theoretical voltage (E th) decreases once the battery is connected to an
external circuit, and we must now calculate the actual voltage of the fuel
cell. To do this, several phenomena must be considered such as the kinetics
of the electrodes, the activation overpotential, and the currents and potentials
that appear in the cell [OHA 09]. These phenomena describe the rate of the
electrochemical reaction as well as the energy losses.
2.4.1. Electrode kinetics
When a metal electrode is in contact with an electrolyte, a characteristic
potential difference is created between the internal potential of the electrode
and the internal potential of the electrolyte.