Page 155 - Introduction to chemical reaction engineering and kinetics
P. 155
6.4 Simple Collision Theory of Reaction Rates 137
8.5
8.Oi
0 0.01 0.02 0.03 0.04 0.05 0.06 0
P,-'/kPa-'
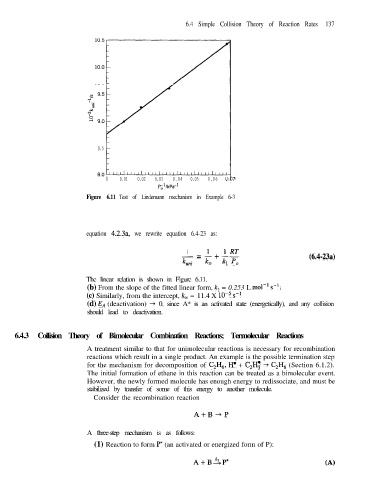
Figure 6.11 Test of Lindemann mechanism in Example 6-3
equation 4.2.3a, we rewrite equation 6.4-23 as:
1
-= $+L!Z (6.4-23a)
kuni m kl po
The linear relation is shown in Figure 6.11.
(b) From the slope of the fitted linear form, k, = 0.253 L mol-’ s-l.
(c) Similarly, from the intercept, km = 11.4 X lop5 s-l
(d) EA (deactivation) -+ 0, since A* is an activated state (energetically), and any collision
should lead to deactivation.
6.4.3 Collision Theory of Bimolecular Combination Reactions; Termolecular Reactions
A treatment similar to that for unimolecular reactions is necessary for recombination
reactions which result in a single product. An example is the possible termination step
for the mechanism for decomposition of C$H,, Ho + %HT -+ C,H, (Section 6.1.2).
The initial formation of ethane in this reaction can be treated as a bimolecular event.
However, the newly formed molecule has enough energy to redissociate, and must be
stabilized by transfer of some of this energy to another molecule.
Consider the recombination reaction
A+B+P
A three-step mechanism is as follows:
(1) Reaction to form P* (an activated or energized form of P):
A+Bk’-P* (4