Page 158 - Introduction to chemical reaction engineering and kinetics
P. 158
140 Chapter 6: Fundamentals of Reaction Rates
Reactants K* Products
AB+C- -J-f+ A + B C
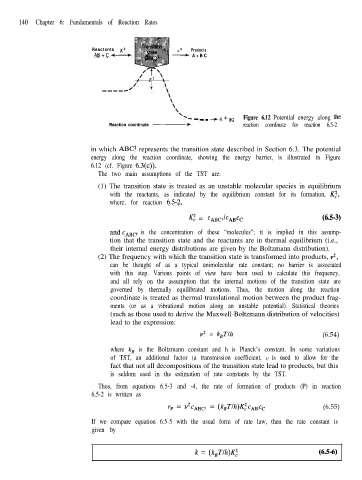
-c _ _ _ + A + Bc Figure 6.12 Potential energy along the
Reaction coordinate - reaction coordinate for reaction 6.5-2
in which ABC’ represents the transition state described in Section 6.3. The potential
energy along the reaction coordinate, showing the energy barrier, is illustrated in Figure
6.12 (cf. Figure 6.3(c)).
The two main assumptions of the TST are:
(1) The transition state is treated as an unstable molecular species in equilibrium
with the reactants, as indicated by the equilibrium constant for its formation, Kz,
where, for reaction 6.5-2,
K: = cAB~tlcABc~ (6.53)
and CABCI is the concentration of these “molecules”; it is implied in this assump-
tion that the transition state and the reactants are in thermal equilibrium (i.e.,
their internal energy distributions are given by the Boltzmann distribution).
(2) The frequency with which the transition state is transformed into products, vt,
can be thought of as a typical unimolecular rate constant; no barrier is associated
with this step. Various points of view have been used to calculate this frequency,
and all rely on the assumption that the internal motions of the transition state are
governed by thermally equilibrated motions. Thus, the motion along the reaction
coordinate is treated as thermal translational motion between the product frag-
ments (or as a vibrational motion along an unstable potential). Statistical theories
(such as those used to derive the Maxwell-Boltzmann distribution of velocities)
lead to the expression:
vs = k,Tlh (6.54)
where k, is the Boltzmann constant and h is Planck’s constant. In some variations
of TST, an additional factor (a transmission coefficient, K) is used to allow for the
fact that not all decompositions of the transition state lead to products, but this
is seldom used in the estimation of rate constants by the TST.
Thus, from equations 6.5-3 and -4, the rate of formation of products (P) in reaction
6.5-2 is written as
(6.55)
If we compare equation 6.5-5 with the usual form of rate law, then the rate constant is
given by