Page 244 - Introduction to chemical reaction engineering and kinetics
P. 244
226 Chapter 9: Multiphase Reacting Systems
Unreacted solid
/
/
I
\
\
Solid & Gas Solid &+ Gas Solid A Gas
Profile Profile
(a) Nonporous (b) Moderately porous (c) Very porous
B particle B particle B particle
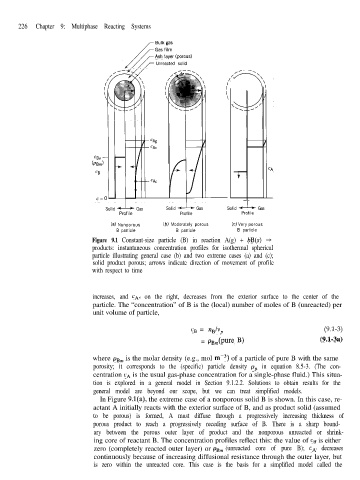
Figure 9.1 Constant-size particle (B) in reaction A(g) + bB(s) +
products: instantaneous concentration profiles for isothermal spherical
particle illustrating general case (b) and two extreme cases (a) and (c);
solid product porous; arrows indicate direction of movement of profile
with respect to time
increases, and cA, on the right, decreases from the exterior surface to the center of the
particle. The “concentration” of B is the (local) number of moles of B (unreacted) per
unit volume of particle,
cB = nBlvp (9.1-3)
= PBm(pure B) (9.1-3a)
where pBm is the molar density (e.g., mol mP3) of a particle of pure B with the same
porosity; it corresponds to the (specific) particle density pP in equation 8.5-3. (The con-
centration cA is the usual gas-phase concentration for a single-phase fluid.) This situa-
tion is explored in a general model in Section 9.1.2.2. Solutions to obtain results for the
general model are beyond our scope, but we can treat simplified models.
In Figure 9.l(a), the extreme case of a nonporous solid B is shown. In this case, re-
actant A initially reacts with the exterior surface of B, and as product solid (assumed
to be porous) is formed, A must diffuse through a progressively increasing thickness of
porous product to reach a progressively receding surface of B. There is a sharp bound-
ary between the porous outer layer of product and the nonporous unreacted or shrink-
ing core of reactant B. The concentration profiles reflect this: the value of cn is either
zero (completely reacted outer layer) or pBm (unreacted core of pure B); cA decreases
continuously because of increasing diffusional resistance through the outer layer, but
is zero within the unreacted core. This case is the basis for a simplified model called the