Page 273 - Introduction to chemical reaction engineering and kinetics
P. 273
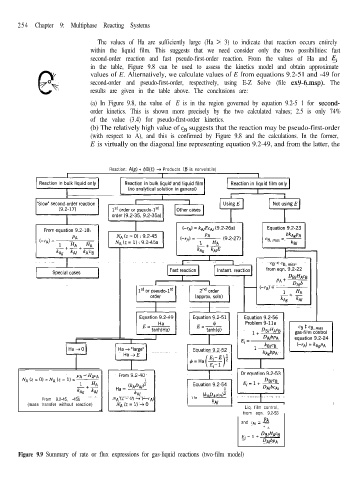
254 Chapter 9: Multiphase Reacting Systems
The values of Ha are sufficiently large (Ha > 3) to indicate that reaction occurs entirely
within the liquid film. This suggests that we need consider only the two possibilities: fast
v
second-order reaction and fast pseudo-first-order reaction. From the values of Ha and Ei
7O-v
0 in the table, Figure 9.8 can be used to assess the kinetics model and obtain approximate
values of E. Alternatively, we calculate values of E from equations 9.2-51 and -49 for
second-order and pseudo-first-order, respectively, using E-Z Solve (file ex9-6.msp).
The
results are given in the table above. The conclusions are:
(a) In Figure 9.8, the value of E is in the region governed by equation 9.2-5 1 for second-
order kinetics. This is shown more precisely by the two calculated values; 2.5 is only 74%
of the value (3.4) for pseudo-first-order kinetics.
(b) The relatively high value of cn suggests that the reaction may be pseudo-first-order
(with respect to A), and this is confirmed by Figure 9.8 and the calculations. In the former,
E is virtually on the diagonal line representing equation 9.2-49, and from the latter, the
Reaction: A(g) + bB(O + Products (B is nonvolatile)
From 9.2-45, -45a
(mass transfer without reaction) .., .I ^ I .-
’ Liq. film control,
from eqn. 9.2-53 I
PA
and cAi = -
* A
DBI*A~B
Ei = 1 + -
*/vbPn
Figure 9.9 Summary of rate or flux expressions for gas-liquid reactions (two-film model)