Page 316 - Introduction to chemical reaction engineering and kinetics
P. 316
12.3 Design Equations for a Batch Reactor 297
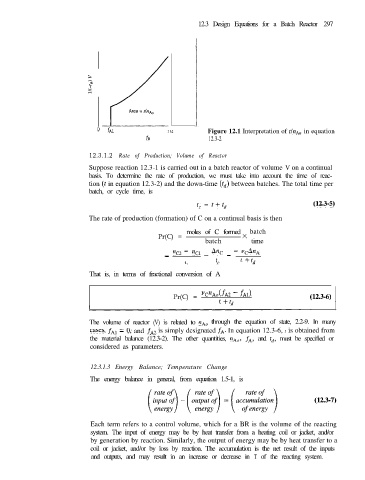
o fA1 fA2 Figure 12.1 Interpretation of tInA in equation
fA 12.3-2
12.3.1.2 Rate of Production; Volume of Reactor
Suppose reaction 12.3-1 is carried out in a batch reactor of volume V on a continual
basis. To determine the rate of production, we must take into account the time of reac-
tion (t in equation 12.3-2) and the down-time (td) between batches. The total time per
batch, or cycle time, is
t, = t+t, (12.36)
The rate of production (formation) of C on a continual basis is then
moles of C formed x batch
Pr(C) =
batch time
= %2 - %I _ Aac _ - lkAnA
-
-
-
t, tc t + td
That is, in terms of fractional conversion of A
Pr(C) = %IzAo(fA2 - fAd
t + td
The volume of reactor (V) is related to nAO through the equation of state, 2.2-9. In many
Cases, fAi = 0, and f,2 is simply designated fA. In equation 12.3-6, t is obtained from
the material balance (12.3-2). The other quantities, nAO, fA, and td, must be specified or
considered as parameters.
12.3.1.3 Energy Balance; Temperature Change
The energy balance in general, from equation 1.5-1, is
Each term refers to a control volume, which for a BR is the volume of the reacting
system. The input of energy may be by heat transfer from a heating coil or jacket, and/or
by generation by reaction. Similarly, the output of energy may be by heat transfer to a
coil or jacket, and/or by loss by reaction. The accumulation is the net result of the inputs
and outputs, and may result in an increase or decrease in T of the reacting system.