Page 93 - Laboratory Manual in Physical Geology
P. 93
Tetrahedron
Needles
(acicular) (4 faces)
Twinned
Pyramidal EQUANT
Cube
(6 faces)
Bladed
Dodecahedron
(12 faces)
Pyritohedron
Octahedron (12 faces)
((dipyramid)) Dendritic
Tabular (shaped Fibrous
like a book)
Radiating needles
Rhombohedron
(a leaning block with Prismatic Wires Botryoidal (bubbly
6 faces, each a rhombus) masses; radiating
Dipyramid prism Scalenohedrons needles inside)
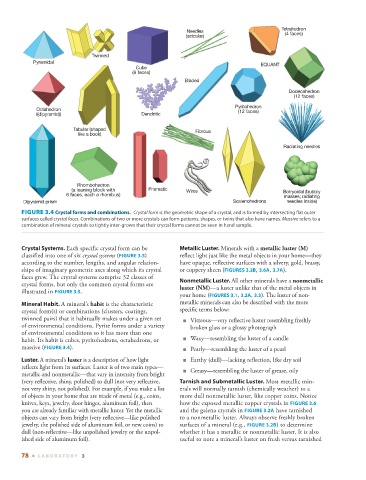
FIGURE 3.4 Crystal forms and combinations. Crystal form is the geometric shape of a crystal, and is formed by intersecting flat outer
surfaces called crystal faces . Combinations of two or more crystals can form patterns, shapes, or twins that also have names. Massive refers to a
combination of mineral crystals so tightly inter-grown that their crystal forms cannot be seen in hand sample.
Crystal Systems. Each specific crystal form can be Metallic Luster. Minerals with a metallic luster (M)
classified into one of six crystal systems ( FIGURE 3.5 ) reflect light just like the metal objects in your home—they
according to the number, lengths, and angular relation- have opaque, reflective surfaces with a silvery, gold, brassy,
ships of imaginary geometric axes along which its crystal or coppery sheen ( FIGURES 3.2B , 3.6A , 3.7A ).
faces grew. The crystal systems comprise 32 classes of Nonmetallic Luster. All other minerals have a nonmetallic
crystal forms, but only the common crystal forms are
luster (NM) —a luster unlike that of the metal objects in
illustrated in FIGURE 3.5 .
your home ( FIGURES 3.1 , 3.2A , 3.3 ). The luster of non-
Mineral Habit. A mineral’s habit is the characteristic metallic minerals can also be described with the more
crystal form(s) or combinations (clusters, coatings, specific terms below:
twinned pairs) that it habitually makes under a given set ■ Vitreous—very reflective luster resembling freshly
of environmental conditions. Pyrite forms under a variety broken glass or a glossy photograph
of environmental conditions so it has more than one
habit. Its habit is cubes, pyritohedrons, octahedrons, or ■ Waxy—resembling the luster of a candle
massive ( FIGURE 3.4 ). ■ Pearly—resembling the luster of a pearl
Luster. A mineral’s luster is a description of how light ■ Earthy (dull)—lacking reflection, like dry soil
reflects light from its surfaces. Luster is of two main types— ■ Greasy—resembling the luster of grease, oily
metallic and nonmetallic—that vary in intensity from bright
(very reflective, shiny, polished) to dull (not very reflective, Tarnish and Submetallic Luster. Most metallic min-
not very shiny, not polished). For example, if you make a list erals will normally tarnish (chemically weather) to a
of objects in your home that are made of metal (e.g., coins, more dull nonmetallic luster, like copper coins. Notice
knives, keys, jewelry, door hinges, aluminum foil), then how the exposed metallic copper crystals in FIGURE 3.6
you are already familiar with metallic luster. Yet the metallic and the galena crystals in FIGURE 3.2A have tarnished
objects can vary from bright (very reflective—like polished to a nonmetallic luster. Always observe freshly broken
jewelry, the polished side of aluminum foil, or new coins) to surfaces of a mineral (e.g., FIGURE 3.2B ) to determine
dull (non-reflective—like unpolished jewelry or the unpol- whether it has a metallic or nonmetallic luster. It is also
ished side of aluminum foil). useful to note a mineral’s luster on fresh versus tarnished
78 ■ L ABOR ATORY 3