Page 101 - Lindens Handbook of Batteries
P. 101
3.20 PRINCIPLES OF OPERATION
0.8
0.7
Li/MnO 2
0.6
Zn/HgO
0.5
Wh/cm 3 0.4 Zn/Ag O
2
0.3
0.2
0.1
0
0 0.2 0.4 0.6 0.8 1.0 1.2
Volume, cm 3
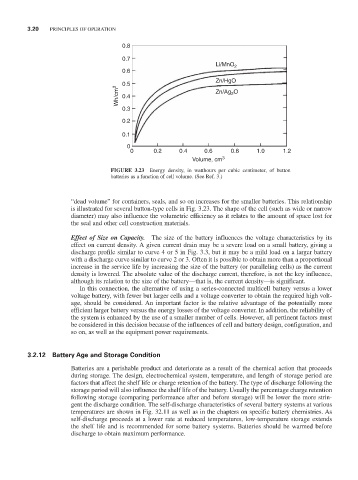
FIGURE 3.23 Energy density, in watthours per cubic centimeter, of button
batteries as a function of cell volume. (See Ref. 3.)
“dead volume” for containers, seals, and so on increases for the smaller batteries. This relationship
is illustrated for several button-type cells in Fig. 3.23. The shape of the cell (such as wide or narrow
diameter) may also influence the volumetric efficiency as it relates to the amount of space lost for
the seal and other cell construction materials.
Effect of Size on Capacity. The size of the battery influences the voltage characteristics by its
effect on current density. A given current drain may be a severe load on a small battery, giving a
discharge profile similar to curve 4 or 5 in Fig. 3.3, but it may be a mild load on a larger battery
with a discharge curve similar to curve 2 or 3. Often it is possible to obtain more than a proportional
increase in the service life by increasing the size of the battery (or paralleling cells) as the current
density is lowered. The absolute value of the discharge current, therefore, is not the key influence,
although its relation to the size of the battery—that is, the current density—is significant.
In this connection, the alternative of using a series-connected multicell battery versus a lower
voltage battery, with fewer but larger cells and a voltage converter to obtain the required high volt-
age, should be considered. An important factor is the relative advantage of the potentially more
efficient larger battery versus the energy losses of the voltage converter. In addition, the reliability of
the system is enhanced by the use of a smaller number of cells. However, all pertinent factors must
be considered in this decision because of the influences of cell and battery design, configuration, and
so on, as well as the equipment power requirements.
3.2.12 Battery Age and Storage Condition
Batteries are a perishable product and deteriorate as a result of the chemical action that proceeds
during storage. The design, electrochemical system, temperature, and length of storage period are
factors that affect the shelf life or charge retention of the battery. The type of discharge following the
storage period will also influence the shelf life of the battery. Usually the percentage charge retention
following storage (comparing performance after and before storage) will be lower the more strin-
gent the discharge condition. The self-discharge characteristics of several battery systems at various
temperatures are shown in Fig. 32.11 as well as in the chapters on specific battery chemistries. As
self-discharge proceeds at a lower rate at reduced temperatures, low-temperature storage extends
the shelf life and is recommended for some battery systems. Batteries should be warmed before
discharge to obtain maximum performance.