Page 191 - Lindens Handbook of Batteries
P. 191
8.4 PRIMARY BATTERIES
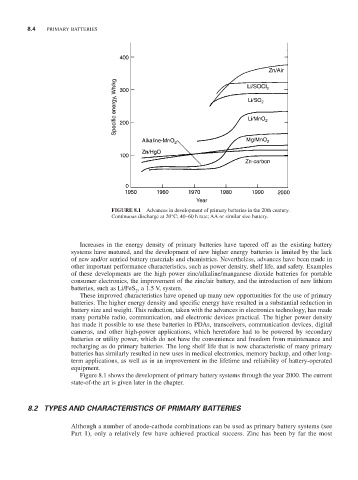
FIGURE 8.1 Advances in development of primary batteries in the 20th century.
Continuous discharge at 20°C; 40–60 h rate; AA or similar size battery.
Increases in the energy density of primary batteries have tapered off as the existing battery
systems have matured, and the development of new higher energy batteries is limited by the lack
of new and/or untried battery materials and chemistries. Nevertheless, advances have been made in
other important performance characteristics, such as power density, shelf life, and safety. Examples
of these developments are the high power zinc/alkaline/manganese dioxide batteries for portable
consumer electronics, the improvement of the zinc/air battery, and the introduction of new lithium
batteries, such as Li/FeS , a 1.5 V, system.
2
These improved characteristics have opened up many new opportunities for the use of primary
batteries. The higher energy density and specific energy have resulted in a substantial reduction in
battery size and weight. This reduction, taken with the advances in electronics technology, has made
many portable radio, communication, and electronic devices practical. The higher power density
has made it possible to use these batteries in PDAs, transceivers, communication devices, digital
cameras, and other high-power applications, which heretofore had to be powered by secondary
batteries or utility power, which do not have the convenience and freedom from maintenance and
recharging as do primary batteries. The long shelf life that is now characteristic of many primary
batteries has similarly resulted in new uses in medical electronics, memory backup, and other long-
term applications, as well as in an improvement in the lifetime and reliability of battery-operated
equipment.
Figure 8.1 shows the development of primary battery systems through the year 2000. The current
state-of-the art is given later in the chapter.
8.2 TYPES AND CHARACTERISTICS OF PRIMARY BATTERIES
Although a number of anode-cathode combinations can be used as primary battery systems (see
Part 1), only a relatively few have achieved practical success. Zinc has been by far the most