Page 195 - Lindens Handbook of Batteries
P. 195
8.8 PRIMARY BATTERIES
8.3 COMPARISON OF THE PERFORMANCE CHARACTERISTICS OF
PRIMARY BATTERY SYSTEMS
8.3.1 General
A qualitative comparison of the various primary battery systems is given in Table 8.2. This listing
illustrates the performance advantages of the lithium anode batteries. Nevertheless, the conventional
primary batteries, because of their low cost, availability, and generally acceptable performance in
many consumer applications, still maintain a major share of the market.
The characteristics of the major primary batteries are summarized in Table 8.3. This table is
supplemented by Table 1.2 in Chap. 1, which lists the theoretical and practical electrical charac-
teristics of these primary battery systems. A graphic comparison of the theoretical and practical
performance of various battery systems given in Fig. 1.4 shows that only about 25 to 35% of
the theoretical capacity is attained under practical conditions as a result of design and discharge
requirements.
It should be noted, as discussed in detail in Chaps. 1, 3 and 32, that most of these types of data and
comparisons are based on the performance characteristics of single-cell batteries and are necessarily
approximations, with each system presented under favorable discharge conditions. The specific per-
formance of a battery system is very dependent on the cell and battery design and all of the specific
conditions of use and discharge of the battery.
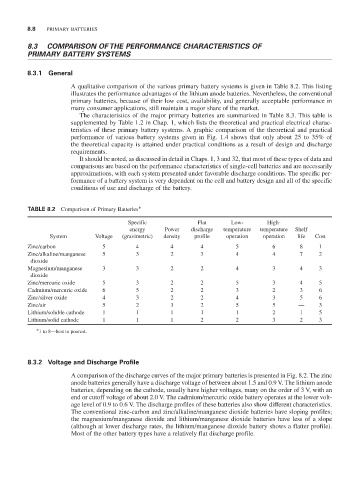
TABLE 8.2 Comparison of Primary Batteries*
Specific Flat Low- High-
energy Power discharge temperature temperature Shelf
System Voltage (gravimetric) density profile operation operation life Cost
Zinc/carbon 5 4 4 4 5 6 8 1
Zinc/alkaline/manganese 5 3 2 3 4 4 7 2
dioxide
Magnesium/manganese 3 3 2 2 4 3 4 3
dioxide
Zinc/mercuric oxide 5 3 2 2 5 3 4 5
Cadmium/mercuric oxide 6 5 2 2 3 2 3 6
Zinc/silver oxide 4 3 2 2 4 3 5 6
Zinc/air 5 2 3 2 5 5 — 3
Lithium/soluble cathode 1 1 1 1 1 2 1 5
Lithium/solid cathode 1 1 1 2 2 3 2 3
*1 to 8—best to poorest.
8.3.2 Voltage and Discharge Profile
A comparison of the discharge curves of the major primary batteries is presented in Fig. 8.2. The zinc
anode batteries generally have a discharge voltage of between about 1.5 and 0.9 V. The lithium anode
batteries, depending on the cathode, usually have higher voltages, many on the order of 3 V, with an
end or cutoff voltage of about 2.0 V. The cadmium/mercuric oxide battery operates at the lower volt-
age level of 0.9 to 0.6 V. The discharge profiles of these batteries also show different characteristics.
The conventional zinc-carbon and zinc/alkaline/manganese dioxide batteries have sloping profiles;
the magnesium/manganese dioxide and lithium/manganese dioxide batteries have less of a slope
(although at lower discharge rates, the lithium/manganese dioxide battery shows a flatter profile).
Most of the other battery types have a relatively flat discharge profile.