Page 208 - Lindens Handbook of Batteries
P. 208
9.2 PRIMARY BATTERIES
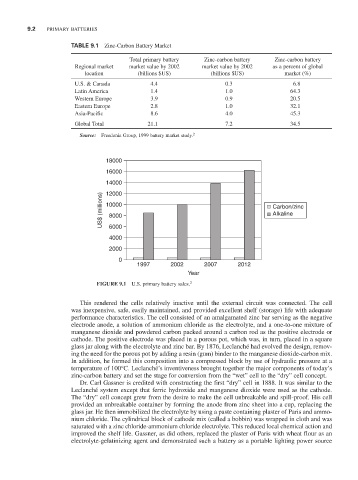
TABLE 9.1 Zinc-Carbon Battery Market
Total primary battery Zinc-carbon battery Zinc-carbon battery
Regional market market value by 2002 market value by 2002 as a percent of global
location (billions $US) (billions $US) market (%)
U.S. & Canada 4.4 0.3 6.8
Latin America 1.4 1.0 64.3
Western Europe 3.9 0.9 20.5
Eastern Europe 2.8 1.0 32.1
Asia-Pacific 8.6 4.0 45.3
Global Total 21.1 7.2 34.5
Source: Freedonia Group, 1999 battery market study. 2
18000
16000
14000
12000
US$ (millions) 10000 Carbon/zinc
Alkaline
8000
6000
4000
2000
0
1997 2002 2007 2012
Year
FIGURE 9.1 U.S. primary battery sales. 2
This rendered the cells relatively inactive until the external circuit was connected. The cell
was inexpensive, safe, easily maintained, and provided excellent shelf (storage) life with adequate
performance characteristics. The cell consisted of an amalgamated zinc bar serving as the negative
electrode anode, a solution of ammonium chloride as the electrolyte, and a one-to-one mixture of
manganese dioxide and powdered carbon packed around a carbon rod as the positive electrode or
cathode. The positive electrode was placed in a porous pot, which was, in turn, placed in a square
glass jar along with the electrolyte and zinc bar. By 1876, Leclanché had evolved the design, remov-
ing the need for the porous pot by adding a resin (gum) binder to the manganese dioxide-carbon mix.
In addition, he formed this composition into a compressed block by use of hydraulic pressure at a
temperature of 100°C. Leclanché’s inventiveness brought together the major components of today’s
zinc-carbon battery and set the stage for conversion from the “wet” cell to the “dry” cell concept.
Dr. Carl Gassner is credited with constructing the first “dry” cell in 1888. It was similar to the
Leclanché system except that ferric hydroxide and manganese dioxide were used as the cathode.
The “dry” cell concept grew from the desire to make the cell unbreakable and spill-proof. His cell
provided an unbreakable container by forming the anode from zinc sheet into a cup, replacing the
glass jar. He then immobilized the electrolyte by using a paste containing plaster of Paris and ammo-
nium chloride. The cylindrical block of cathode mix (called a bobbin) was wrapped in cloth and was
saturated with a zinc chloride-ammonium chloride electrolyte. This reduced local chemical action and
improved the shelf life. Gassner, as did others, replaced the plaster of Paris with wheat flour as an
electrolyte-gelatinizing agent and demonstrated such a battery as a portable lighting power source