Page 178 - Lindens Handbook of Batteries
P. 178
BATTERY ELECTROLYTES 7.3
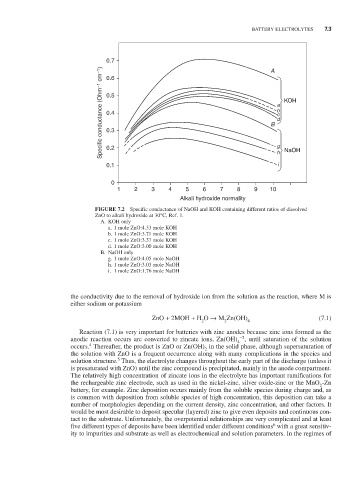
0.7 A
Specific conductance (Ohm –1 cm –1 ) 0.5 B a b c d KOH
0.6
0.4
0.3
0.2
0.1 g h i NaOH
0
1 2 3 4 5 6 7 8 9 10
Alkali hydroxide normality
FIGURE 7.2 Specific conductance of NaOH and KOH containing different ratios of dissolved
ZnO to alkali hydroxide at 30°C, Ref. 1.
A. KOH only
a. 1 mole ZnO:4.33 mole KOH
b. 1 mole ZnO:3.71 mole KOH
c. 1 mole ZnO:3.37 mole KOH
d. 1 mole ZnO:3.00 mole KOH
B. NaOH only
g. 1 mole ZnO:4.05 mole NaOH
h. 1 mole ZnO:3.03 mole NaOH
i. 1 mole ZnO:1.76 mole NaOH
the conductivity due to the removal of hydroxide ion from the solution as the reaction, where M is
either sodium or potassium
ZnO + 2MOH + H O → M Zn(OH) (7.1)
2 2 4
Reaction (7.1) is very important for batteries with zinc anodes because zinc ions formed as the
anodic reaction occurs are converted to zincate ions, Zn(OH) 4 -2 , until saturation of the solution
4
occurs. Thereafter, the product is ZnO or Zn(OH) in the solid phase, although supersaturation of
2
the solution with ZnO is a frequent occurrence along with many complications in the species and
solution structure. Thus, the electrolyte changes throughout the early part of the discharge (unless it
5
is presaturated with ZnO) until the zinc compound is precipitated, mainly in the anode compartment.
The relatively high concentration of zincate ions in the electrolyte has important ramifications for
the rechargeable zinc electrode, such as used in the nickel-zinc, silver oxide-zinc or the MnO -Zn
2
battery, for example. Zinc deposition occurs mainly from the soluble species during charge and, as
is common with deposition from soluble species of high concentration, this deposition can take a
number of morphologies depending on the current density, zinc concentration, and other factors. It
would be most desirable to deposit specular (layered) zinc to give even deposits and continuous con-
tact to the substrate. Unfortunately, the overpotential relationships are very complicated and at least
6
five different types of deposits have been identified under different conditions with a great sensitiv-
ity to impurities and substrate as well as electrochemical and solution parameters. In the regimes of