Page 235 - Lindens Handbook of Batteries
P. 235
ZINC-CARBON BATTERIES—LECLANCHÉ AND ZINC CHLORIDE CELL SYSTEMS 9.29
TABLE 9.6 Flash Current and Internal Resistance for Various
Battery Sizes
Typical maximum flash Approximate internal
current, A resistance, ohms
Size LC* ZC* LC ZC
N 2.5 ... 0.6 ...
AAA 3 4 0.4 0.35
AA 4 5 0.30 0.28
C 5 7 0.39 0.23
D 6 9 0.27 0.18
F 9 11 0.17 0.13
9 V (battery) 0.6 0.8 5 4.5
*LC: Leclanché, ZC: Zinc chloride.
Source: Eveready Battery Engineering Data. 12
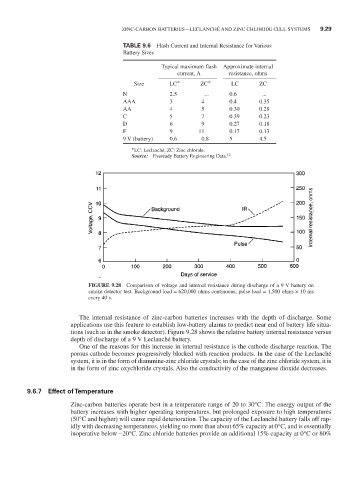
FIGURE 9.28 Comparison of voltage and internal resistance during discharge of a 9 V battery on
smoke detector test. Background load = 620,000 ohms continuous; pulse load = 1,500 ohms × 10 ms
every 40 s.
The internal resistance of zinc-carbon batteries increases with the depth of discharge. Some
applications use this feature to establish low-battery alarms to predict near end of battery life situa-
tions (such as in the smoke detector). Figure 9.28 shows the relative battery internal resistance versus
depth of discharge of a 9 V Leclanché battery.
One of the reasons for this increase in internal resistance is the cathode discharge reaction. The
porous cathode becomes progressively blocked with reaction products. In the case of the Leclanché
system, it is in the form of diammine-zinc chloride crystals; in the case of the zinc chloride system, it is
in the form of zinc oxychloride crystals. Also the conductivity of the manganese dioxide decreases.
9.6.7 Effect of Temperature
Zinc-carbon batteries operate best in a temperature range of 20 to 30°C. The energy output of the
battery increases with higher operating temperatures, but prolonged exposure to high temperatures
(50°C and higher) will cause rapid deterioration. The capacity of the Leclanché battery falls off rap-
idly with decreasing temperatures, yielding no more than about 65% capacity at 0°C, and is essentially
inoperative below -20°C. Zinc chloride batteries provide an additional 15% capacity at 0°C or 80%