Page 236 - Lindens Handbook of Batteries
P. 236
9.30 PRIMARY BATTERIES
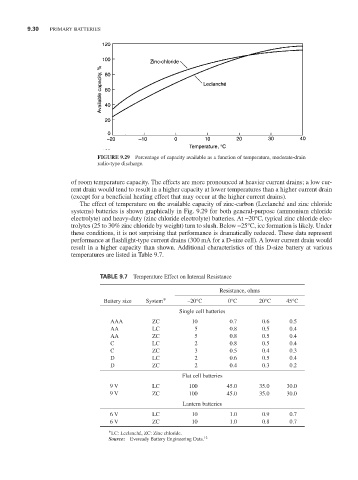
FIGURE 9.29 Percentage of capacity available as a function of temperature, moderate-drain
radio-type discharge.
of room temperature capacity. The effects are more pronounced at heavier current drains; a low cur-
rent drain would tend to result in a higher capacity at lower temperatures than a higher current drain
(except for a beneficial heating effect that may occur at the higher current drains).
The effect of temperature on the available capacity of zinc-carbon (Leclanché and zinc chloride
systems) batteries is shown graphically in Fig. 9.29 for both general-purpose (ammonium chloride
electrolyte) and heavy-duty (zinc chloride electrolyte) batteries. At -20°C, typical zinc chloride elec-
trolytes (25 to 30% zinc chloride by weight) turn to slush. Below -25°C, ice formation is likely. Under
these conditions, it is not surprising that performance is dramatically reduced. These data represent
performance at flashlight-type current drains (300 mA for a D-size cell). A lower current drain would
result in a higher capacity than shown. Additional characteristics of this D-size battery at various
temperatures are listed in Table 9.7.
TABLE 9.7 Temperature Effect on Internal Resistance
Resistance, ohms
Battery size System* –20°C 0°C 20°C 45°C
Single cell batteries
AAA ZC 10 0.7 0.6 0.5
AA LC 5 0.8 0.5 0.4
AA ZC 5 0.8 0.5 0.4
C LC 2 0.8 0.5 0.4
C ZC 3 0.5 0.4 0.3
D LC 2 0.6 0.5 0.4
D ZC 2 0.4 0.3 0.2
Flat cell batteries
9 V LC 100 45.0 35.0 30.0
9 V ZC 100 45.0 35.0 30.0
Lantern batteries
6 V LC 10 1.0 0.9 0.7
6 V ZC 10 1.0 0.8 0.7
*LC: Leclanché, ZC: Zinc chloride.
Source: Eveready Battery Engineering Data. 12