Page 40 - Lindens Handbook of Batteries
P. 40
BASIC CONCEPTS 1.15
10000
Theoretical specific energy
active materials
Theoretical specific energy
practical battery
Actual specific energy
Specific energy (Wh/kg) 100
1000
10 Zinc/air
Leclanche dry cell Alkaline/MnO 2 Lithium/MnO 2 Lithium/SO 2 Lead-acid Nickel-cadmium Nickel-metal hydride Lithium-ion
Primary batteries Rechargeable batteries
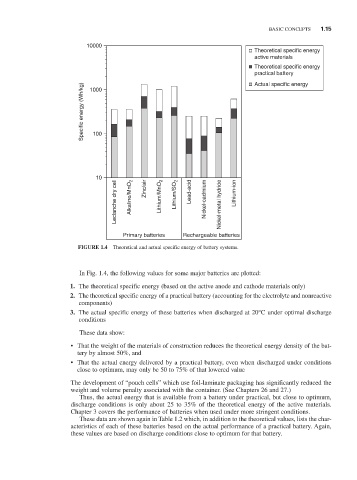
FIGURE 1.4 Theoretical and actual specific energy of battery systems.
In Fig. 1.4, the following values for some major batteries are plotted:
1. The theoretical specific energy (based on the active anode and cathode materials only)
2. The theoretical specific energy of a practical battery (accounting for the electrolyte and nonreactive
components)
3. The actual specific energy of these batteries when discharged at 20°C under optimal discharge
conditions
These data show:
• That the weight of the materials of construction reduces the theoretical energy density of the bat-
tery by almost 50%, and
• That the actual energy delivered by a practical battery, even when discharged under conditions
close to optimum, may only be 50 to 75% of that lowered value
The development of “pouch cells” which use foil-laminate packaging has significantly reduced the
weight and volume penalty associated with the container. (See Chapters 26 and 27.)
Thus, the actual energy that is available from a battery under practical, but close to optimum,
discharge conditions is only about 25 to 35% of the theoretical energy of the active materials.
Chapter 3 covers the performance of batteries when used under more stringent conditions.
These data are shown again in Table 1.2 which, in addition to the theoretical values, lists the char-
acteristics of each of these batteries based on the actual performance of a practical battery. Again,
these values are based on discharge conditions close to optimum for that battery.