Page 42 - Lindens Handbook of Batteries
P. 42
BASIC CONCEPTS 1.17
350
Lithium
300
250
Specific energy (Wh/kg) 200 Alkaline- Lithium-ion
150
MnO
2
MnO
High
2
100
performance Alkaline-
Leclanché Ni-MH
50 Lead-acidNi-Cd
Leclanché
0
1946 1955 1965 1985 2010 1940 1955 1985 2010
Primary batteries Secondary batteries
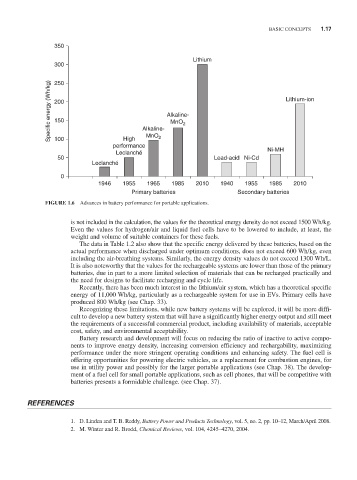
FIGURE 1.6 Advances in battery performance for portable applications.
is not included in the calculation, the values for the theoretical energy density do not exceed 1500 Wh/kg.
Even the values for hydrogen/air and liquid fuel cells have to be lowered to include, at least, the
weight and volume of suitable containers for these fuels.
The data in Table 1.2 also show that the specific energy delivered by these batteries, based on the
actual performance when discharged under optimum conditions, does not exceed 600 Wh/kg, even
including the air-breathing systems. Similarly, the energy density values do not exceed 1300 Wh/L.
It is also noteworthy that the values for the rechargeable systems are lower than those of the primary
batteries, due in part to a more limited selection of materials that can be recharged practically and
the need for designs to facilitate recharging and cycle life.
Recently, there has been much interest in the lithium/air system, which has a theoretical specific
energy of 11,000 Wh/kg, particularly as a rechargeable system for use in EVs. Primary cells have
produced 800 Wh/kg (see Chap. 33).
Recognizing these limitations, while new battery systems will be explored, it will be more diffi-
cult to develop a new battery system that will have a significantly higher energy output and still meet
the requirements of a successful commercial product, including availability of materials, acceptable
cost, safety, and environmental acceptability.
Battery research and development will focus on reducing the ratio of inactive to active compo-
nents to improve energy density, increasing conversion efficiency and rechargability, maximizing
performance under the more stringent operating conditions and enhancing safety. The fuel cell is
offering opportunities for powering electric vehicles, as a replacement for combustion engines, for
use in utility power and possibly for the larger portable applications (see Chap. 38). The develop-
ment of a fuel cell for small portable applications, such as cell phones, that will be competitive with
batteries presents a formidable challenge. (see Chap. 37).
REFERENCES
1. D. Linden and T. B. Reddy, Battery Power and Products Technology, vol. 5, no. 2, pp. 10–12, March/April 2008.
2. M. Winter and R. Brodd, Chemical Reviews, vol. 104, 4245–4270, 2004.